Table of Contents:
- Why protein purification matters
- Core principles of protein purification
- Recombinant protein purification methods
- Protein purification workflow
- Applications
With the development of recombinant technology, researchers no longer rely on natural sources for proteins, enzymes, antibodies, or other biomolecules. They can now engineer and produce them in hosts such as E. coli, yeast, or mammalian cells. Recombinant technology has solved the following issues with isolating these biomolecules from natural sources:
- Limited supply
- Impurity
- Batch variability
- Safety risks such as allergic reactions, pathogen transmission, or immune reactions
- Ethical concerns
However, recombinant proteins also contain some critical impurities that are removed through recombinant protein purification.
Why Protein Purification Matters
Recombinant proteins are engineered and produced in well-defined expression systems under controlled conditions. Still, these engineered proteins carry some impurities.
| Impurity | Source | Issue caused |
| Host Cell Proteins (HCPs) | Native proteins from the host that co-purify |
|
| Nucleic Acids (DNA/RNA) | Fragments of host DNA or RNA left after cell lysis |
|
| Endotoxins | Lipopolysaccharides (LPS) |
|
| Protein Aggregates | Misfolded proteins forming insoluble clumps |
|
| Misfolded or Truncated Variants | Incorrectly folded or incomplete recombinant proteins |
|
| Residual Chemicals | Reagents from lysis, purification buffers, or chromatography |
|
These challenges highlight the need for a systematic recombinant protein purification strategy.
Core Principles of Protein Purification
Every protein has unique physical and chemical properties. Protein purification strategies selectively use differences in size, charge, solubility, and binding affinity to separate the target protein from impurities. The purification process follows a standard workflow with the following three phases:
- Capture
- Intermediate purification
- Polishing
The target protein is isolated from the crude mixture in the capture phase. The intermediate purification phase removes most contaminants, and the polishing phase yields highly pure and stable recombinant proteins.
Recombinant Protein Purification Methods
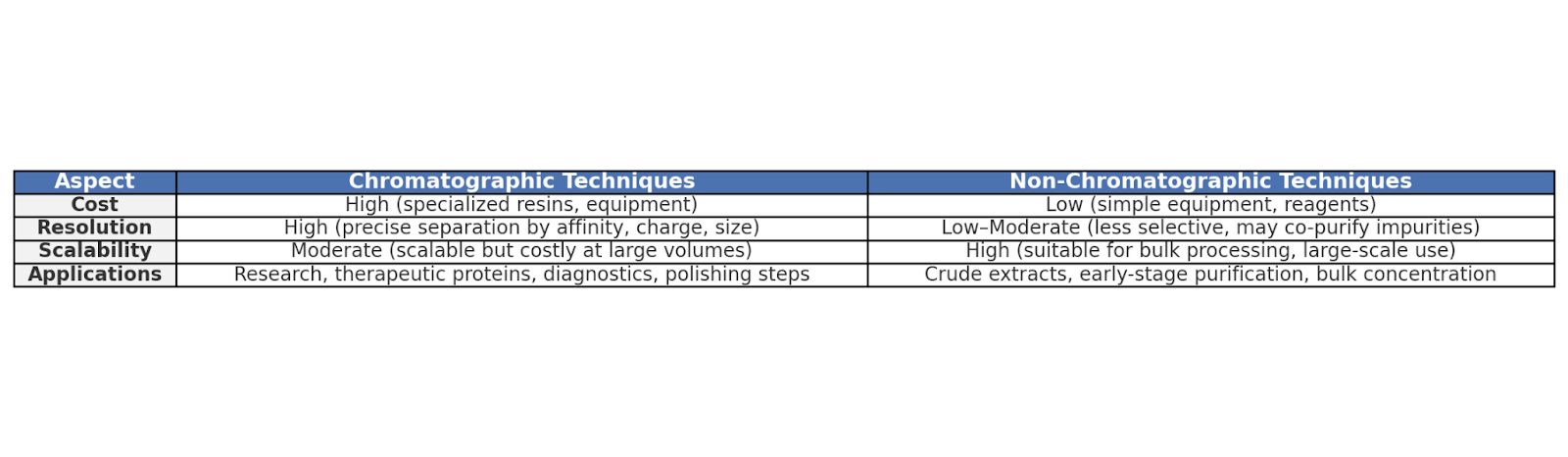
These purification methods are divided into the following two categories:
- Chromatographic Techniques
- Non-Chromatographic Techniques
Chromatographic Techniques
The following are the most commonly used chromatography techniques offering high selectivity and resolution:
Affinity Chromatography
This technique uses specific interactions (such as His-tag/Ni-NTA, GST, or antibody binding) to capture the target protein.
Ion-Exchange Chromatography
This technique uses surface charge differences to separate proteins.
Size-Exclusion Chromatography
Size-exclusion chromatography is used in the final polishing step to remove aggregates by separating proteins based on size.
Non-Chromatographic Techniques
Non-chromatographic techniques are more straightforward and cost-effective techniques used in large-scale or early-stage purification.
Precipitation
This technique uses salts or solvents to make proteins clump together.
Ultrafiltration
This technique utilizes membranes that block proteins, allowing water and small molecules to pass through.
Dialysis
A semi-permeable membrane lets small molecules and buffer components diffuse out, keeping larger proteins inside.
Protein Purification Workflow
The purification process consists of a series of steps to isolate the target protein while removing impurities. Recombinant protein purification includes the following three stages:
- Cell Lysis
- Clarification
- Purification Sequence
Cell Lysis
It is the first step in recombinant protein purification in which the host cells break open to release the expressed protein. It is achieved through the following methods:
- Mechanical methods
- Enzymatic methods
- Chemical methods
The choice of the cell lysis method depends on the host cell type and the sensitivity of the protein to denaturation.
Clarification
After lysis, the crude cell extract contains insoluble materials such as cell debris and nucleic acids. Clarification uses centrifugation or filtration to remove these contaminants. This results in a clarified solution that is suitable for downstream purification.
Purification Sequence
The clarified protein solution undergoes the following purification steps:
Capture
Methods like affinity chromatography are used to separate the target protein from the crude mixture and then increase its concentration.
Intermediate Purification
Techniques such as ion-exchange or hydrophobic interaction chromatography remove most host cell proteins and other impurities.
Polishing
Size-exclusion chromatography removes aggregates, misfolded variants, and remaining contaminants.
Concentration and Storage
Concentration methods, such as ultrafiltration, concentrate the purified protein to the desired level. To maintain structural integrity and activity during long-term storage, proteins may be freeze-dried or stored in buffers containing stabilizers.
Applications
Research, diagnostic, and therapeutic applications use highly purified recombinant proteins for safety, reproducibility, and accurate results.
Biomedical Research
Biomedical research uses recombinant proteins for structural studies, functional assays, and antibody production.
Diagnostics
Recombinant proteins are essential for diagnostic assays like ELISA, rapid tests, and biosensors due to their purity, consistency, and scalability.
Therapeutics
Therapeutic proteins, including hormones, enzymes, and monoclonal antibodies, rely on purification to ensure safety and efficacy.
Industrial and Biotechnological Applications
Recombinant proteins are also used as enzymes in the food and beverage industry, biocatalysts in chemical synthesis, and tools in agricultural biotechnology.
Vaccine Development
Highly purified recombinant proteins are used as antigens in subunit vaccines and research vaccines.